Tel:0086-21-58356513, 58356613
Email:info@skybluechem.com
Site:www.skybluechem.com/
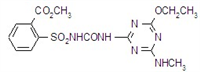
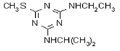
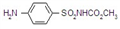
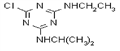
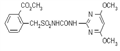
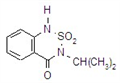
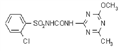
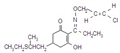
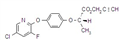
IUPAC name methyl 2-[(4-ethoxy-6-methylamino-1,3,5-triazin-2-yl)carbamoylsulfamoyl]benzoate
Chemical Abstracts name methyl 2-[[[[[4-ethoxy-6-(methylamino)-1,3,5-triazin-2-yl]amino]carbonyl]amino]sulfonyl]benzoate
CAS RN [97780-06-8] methyl ester; [111353-84-5] parent acid Development codes DPX-A7881 (Du Pont)
PHYSICAL CHEMISTRY
Mol. wt. 410.4 M.f. C15H18N6O6S Form Colourless to light tan, odourless crystals. M.p. 194 ºC V.p. 7.73 ´ 10-10 mPa (25 ºC) KOW logP = 0.89 (pH 7), 1.588 (pH 5) Henry 6.34 ´ 10-12 Pa m3 mol-1 (calc.) S.g./density 1.6 Solubility In water 50 mg/l (pH 7, 25 ºC). In acetone 1.6, acetonitrile 0.8, ethanol 0.17, methanol 0.35, methylene chloride 3.9, ethyl acetate 0.68 (all in g/l). Stability Stable at pH 7 and pH 9. Hydrolysis occurs more rapidly at pH 5, DT50 45 d. Photolysis is not a major degradation pathway. Acidic in reaction. pKa 4.6
COMMERCIALISATION
History Herbicide reported by J. R. Stone et al. (Proc. North Cent. Weed Control Conf., 1985, 40, 17). Introduced by E. I. du Pont de Nemours and Co. in 1989. Manufacturers Du Pont
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Uses Post-emergence control of wild mustard, hempnettle and other broad-leaved weeds in oilseed rape, at 15-20 g/ha. Formulation types WG. Selected tradenames: 'Muster' (Du Pont)
ANALYSIS
By hplc. Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >11 000, rabbits >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >5.7 mg/l air. NOEL (90 d) for rats and mice 5000 ppm; (1 y) for rats 500, dogs 3000 ppm; (18 mo) for mice 5000 ppm. Non-oncogenic and non-mutagenic in rats. Non-teratogenic in rats and rabbits. Toxicity class WHO (a.i.) Table 5; EPA (formulation) Not registered in US
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2250 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5620 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish and rainbow trout >600 mg/l. Daphnia LC50 (48 h) 34 mg/l. Bees Acute toxicity to honeybees >12.5 mg/bee. Worms Contact LD50 (14 d) for earthworms >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals Ethametsulfuron-methyl administered to male and female rats was rapidly metabolised and excreted in the urine and faeces. Half-lives for excretion range from 12 hours in male rats to 21-26 hours in female rats. Less than 0.2% of the dose remains in the tissues five days after dosing at the highest dose level. No preferential accumulation of ethametsulfuron-methyl or its metabolites. Plants Oilseed rape was treated with 30 g/ha ethametsulfuron-methyl in a glasshouse: total radiolabelled residues in the foliage decreased rapidly from c. 1.0 ppm immediately after treatment to 0.02 ppm after 31 days; DT50 1-3 h. Two primary metabolites were identified, formed by successive dealkylation; firstly of the ethoxy group, to give the corresponding hydroxytriazine, then of the methylamino substituent. Total radioactive residue in mature oilseed rape was very low (0.008 to 0.012 ppm). No ethametsulfuron-methyl was detected in the seed. Soil/Environment Soil metabolism (aerobic, lab.) DT50 9 w; three major metabolites were identified. In soil photolysis studies, sunlight accelerated degradation three-fold relative to dark controls. Aquatic metabolism (aerobic) DT50 6 mo, (anaerobic) DT50 2-9 mo, depending on sediment pH. In laboratory soil mobility studies based on soil TLC, soil column leaching, and adsorption/desorption studies, mobility is highly dependent on soil characteristics, primary organic matter content and soil pH. Mobility ranges from very mobile in sandy loam soil to very low mobility in loam soil.